

, The Heat Capacity of Carbon Tetrachloride from 15 to 300 K. Carbon tetrachloride (CCl4) is known as a covalent compound because it contains. If both atoms in the bond are nonmetals, it is a covalent bond. Is CCl4 ionic or covalent Carbon is found in group 4 on the periodic table above the heavy 'stair step' line that divides metals and nonmetals, so it is a nonmetal. If one atom in the bond is a metal and the other atom is a nonmetal, it is an ionic bond. Germanium tetrachloride is covalent, just like carbon tetrachloride or silicon tetrachloride. metallic elements or nonmetallic elements.
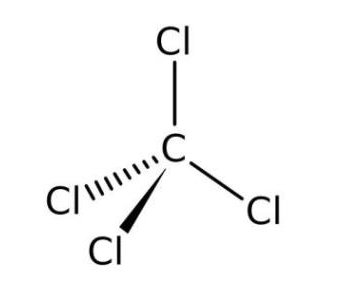
Ionic Liquids Database SRD 156 Clathrate Hydrate Physical Property Database References. Ionic and covalent bonds depend on the nature of the 4 atoms, i.e. Answer (1 of 5): Atomic number of Carbon is 6 and its configuration is 1s2, 2s2 2p2 and its valency is 4. Formula: CCl 4 Molecular weight: 153.823 IUPAC Standard InChI: InChI1S/CCl4/c2-1(3,4). These compounds are often described as having “ionic character” and these types of covalent bonds can often be readily broken to form sets of ions.\) emphasizes that the formulas for molecular compounds are not reduced to their lowest ratios. What is the chemical formula of carbon tetrachloride - Quora. Later in this chapter we will see that many covalent compounds have bonds that are highly polarized with greater electron density around one atom than the other. Nitrogen monoxide (NO) will be a covalently bound molecule (two non-metals), silicon dioxide (SiO 2) will be a covalently bound molecule (a semi-metal and a non-metal) and MgCl 2 will be ionic (a metal and a non-metal). More specifically speaking, carbon tetrachloride is a nonpolar covalent compound because the electrons shared by the carbon and chlorine atoms are nearly at. Thus, the compound formed from sodium and chlorine will be ionic (a metal and a non-metal). To know what types of elements bond to form covalent compounds. Compounds that are composed of only non-metals or semi-metals with non-metals will display covalent bonding and will be classified as molecular compounds. Classify each substance as an atomic element, molecular element, or. Predict whether the compound formed in each case is an ionic or a covalent compound. Carbon Tetrachloride CCl - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities. As a general rule of thumb, compounds that involve a metal binding with either a non-metal or a semi-metal will display ionic bonding. Classify each compound as ionic or molecular.


These groupings are not arbitrary, but are largely based on physical properties and on the tendency of the various elements to bond with other elements by forming either an ionic or a covalent bond. In Chapter 1, we divided the elements in the periodic table into (seemingly) arbitrary groupings the metals, the non-metals, the semi-metals, and so on. The tendency for two or more elements to combine and form a molecule that is stabilized by covalent bonds (a molecular compound) can be predicted simply by the location of the various elements on the periodic table.


 0 kommentar(er)
0 kommentar(er)
